World Nutrition
Volume 3, Number 5, May 2012
Journal of the World Public Health Nutrition Association
Published monthly at www.wphna.org
The Association is an affiliated body of the International Union of Nutritional Sciences For membership and for other contributions, news, columns and services, go to: www.wphna.org
Rio2012. What next
Coming to judgement

Philip James
International Association for the Study of Obesity
London School of Hygiene and Tropical Medicine
Email: jeanhjames@aol.com
Click here for member's profile
Access pdf of this commentary here
Access pdf of associated short communications here
Access pdf of NICE report on public health guidance here
Access pdf of Kumanyika and Economos on evidence here
Introduction
This commentary is about evidence. In particular, it is about what is the best evidence on which judgements, policies and actions can be reliably based, concerning prevention of disease and promotion of good health and well-being, now and in future. This, as I will make clear, is not the same as the best evidence in clinical practice, or for testing the safety of drugs. Public health is more complex.
Judgements, policies and actions should be evidence-based. We all know this. 'Evidence-based medicine' and also 'evidence-based public health' are phrases with mantra-type status. This is curious in a way, for who could disagree? Do any of us believe that any public policies, of any type, should be ignorance-based or prejudice-based? Of course not! We are all for evidence. Knowledge derives from evidence, which is to say, relevant information. This is important for the Rio2012 conference taking place at the end of April as this issue of World Nutrition goes on-line, with its knowledge-policy-action motto. It is all the more important for what comes next – the outcomes of the conference – for action should follow policy analysis and proposals.
The big question is: What counts as 'evidence'? Do all facts contribute, and should they be treated equally? Of course not. The key word above is 'relevant', which in turn often also requires a series of judgements as to what constitutes relevance. In deciding what is knowledge – in our daily lives also – we are all the time making decisions about what information is relevant and what is not relevant, or only marginally so. In other words we are constantly judging what weight to attach to what fact or feature. If as professionals we make the wrong decisions here, we will make wrong judgements, and therefore propose wrong policies.
All of us who see ourselves in any sense as scientists, care about evidence. But in public health and in nutrition, what counts as evidence? What type of organised information is relevant or admissible to our considerations? What is the best kind of evidence? What evidence is most relevant in what context? How much relevant evidence is needed, as a sound basis for judgements? The answers to such questions are not obvious. They are also very important. This is, I suggest, a very big issue for all of us concerned with the use of knowledge for public policy-making and action.
After working for many years as a chair or member of many UK and international committees, I recognise that approaches to assembling and sifting evidence have been and are steadily evolving. But in public health and indeed in nutrition policy-making, it seems to me that professionals in our field have become locked into a mind-set derived from the insistence of physicians that any clinical decision has to be based on very rigid criteria. When this approach is transferred to public health nutrition, it deludes us into imagining that we are little more than beginners at scientific thinking. We are forgetting the success of decades – indeed centuries – of remarkably successful public health investigation, reasoning and initiatives
I am not alone in having these basic concerns. My colleague Association member Shiriki Kumanyika is my successor as one of the chairs of the International Obesity Task Force, For many years she also has been concerned about the types of evidence that are most relevant in prevention of disease and promotion of well-being. For World Nutrition last year, with Christina Economos, she digested the 2010 US Institute of Medicine report Bridging the Gap in Obesity Prevention, produced by a committee which she chaired.(1-4). Its conclusions are along the same lines as set out here. She also writes about the need for policy action in this month's WN. The main text that prompts this commentary is, however, a report produced by the UK National Institute for Health and Clinical Excellence (NICE) (5). It has been a delight to me – and I hope to all who read this.
Box 1
Rio2012: What next
Philip James writes: This note is written in my capacity as an Association Council member, and as one of the Rio2012 participants asked to give a short opening and closing plenary statement. It anticipates one of my proposals.
The motto of our Rio2012 conference is 'knowledge-policy-action'. So how will we continue our work after the conference? As somebody who has struggled since the 1960s to turn modern knowledge of nutrition into enlightened public programmes, I am all for this. But let's be careful. It is one thing to agree a mission statement, and it also one thing to have a meeting – even a big conference – and to develop calls for action. But it is quite another thing to make anything happen in the wide world outside our profession. I write from experience. This includes bitter experience, for the current government of the UK, of which I am a citizen, is now demolishing more of the public health and nutrition structure gradually built up in the last half-century, much of which was retained even in the savage restructuring of the public sector in the 1980s.
So here is my modest proposal, which will come to something only with the sustained and energetic support of colleagues, whether or not they are Association members. Within the Association we agree that nutrition has social, economic and environmental dimensions, as well as biological and behavioural dimensions. We also agree that disease, health and well-being have basic and underlying as well as immediate causes. There is further good reason for us all to agree that public health nutrition is a crucially important part of the whole public health profession and movement.
If you agree with this, then it is time that as a profession we begin to be influential. This task involves not merely commenting on the decisions of others. It means that we ourselves need to set out rational policy lines. I therefore propose that under the general banner of Rio2012. What next that a number of working groups are convened by the Association, whose collective responsibility will be to set a whole new agenda for nutrition and public health in this century, or at least for the next two or three decades, say until 2040.
The report from the UK National Institute for Health and Clinical Evidence (NICE) (5) discussed in this commentary should be a key resource for the Association working groups. Our own reports should include analyses with appropriate graphics to amplify our work. These will give us tools to use in making policy proposals for example on nutritional aspects of women's health, the new Millennium Development Goals starting three years from now in 2015, the prevention of chronic diseases, or nutritional issues in relation to mental health.
I suggest too that the Association will benefit from a specific working group on the topic of this commentary, which in one word, is evidence. Or, more specifically, the scope, nature, variety and quality of evidence needed to come to judgement in our field of public health nutrition. The report of this group should include illuminating examples. These could demonstrate the interactivity of the underlying and basic societal causes of health and disease to politicians and policy-makers, who so often have little idea of the real drivers of our public health problems, or who choose to ignore them.
I hope our response becomes evident in our discussions and agreements that will begin during Rio2012. It must continue afterwards, if we are to be effective in changing the global approach to so many pressing problems.
Evidence, policy and politics
Assessment of evidence for quality, strength and relevance, may sound intellectually tough, and even very technical. It does need focus, and it does have technical aspects. But the whole issue is also political, in the sense of relating to public policy and, as I will show, also in the sense of relating to politicians and vested interests.
The public interest
We can't avoid evidence. Thus, regulations that govern and guide our daily lives are – or should be – based on sound evidence. Here are some examples. Taxation of cigarettes and alcoholic drinks is based at least in part on strong and consistent evidence from many types of study and observation that increased price does help to control and to reduce consumption. Regulation and restriction of drugs, and their occasional withdrawal, follows what should be rigorous and independent evidence rightly relying on scrupulously designed interventions testing for their safety in use – or of unjustified harmful effects. Road speed limits have proved beyond reasonable doubt to reduce death and injury of motorists, passengers and pedestrians. The evidence here includes monitoring of the rates of death and injury after the regulations, in part based on common sense, were put in place. These are some of the reasons for the regulations. The examples given here are obviously in the public interest.
The same should apply to judgements, recommendations and policies concerned with food. A relatively simple example is regulation to ensure that food is safe – usually meaning that any contamination is at levels far below any considered to be possibly harmful. I served on official UK and EU advisory panels on this topic for many years, and the systems worked. Whether they generated the best possible recommendations and policies is another matter.
Now to come to the main topic of this commentary: food, nutrition and health. I have been deeply involved in the issue of evidence in our field, for many years. Thus, I chaired the WHO study group whose '797' report on Diet, Nutrition and the Prevention of Chronic Diseases, was published in 1990 (6). This report made recommendations designed to guide the policies and actions of member states throughout the world. Later I was a member of the next panel that produced the WHO '916' report on the same topic published in 2003 (7). I was also a member of the World Cancer Research Fund panels that produced reports on Food, Nutrition and the Prevention of Cancer from a global perspective, which included policy as well as population and personal recommendations. These reports were published in 1997, 2007 and 2009 (8-10).
When evidence is good enough
With all my colleagues whose work includes membership of independent expert panels, advising national governments or UN agencies and thus member states, I have learned that in all matters of public policy, it is crucial to decide when evidence is good enough to be the basis for action. Thus when I was a young clinician in London in the 1960s, I was caring for terminal cases of lung cancer in young women smokers in a major cancer centre. I also witnessed sudden deaths from heart attacks of many relatively young men, at that time when therapy was fairly primitive. It was obvious that failure by the UK governments of that time to introduce restrictions and disincentives on smoking and on consumption of saturated fat, was in effect a major cause of a very large number of unnecessary deaths.
A little later when working for the Medical Research Council in Jamaica, I had a similar sense of urgency, when as a physician I was caring for malnourished babies at a time when death-rates in babies and young children were high. Happily, the distinguished and socially conscious paediatrician and champion of breastfeeding Derrick Jelliffe, with his wife Eleanore, who worked in many parts of the world including the Caribbean, then began successfully to push for community action.
But when back in the UK and a government advisor, what I found is that some experts are unwilling to agree that evidence is ever adequate as a basis for policy making. I also noticed that those colleagues on UK government advisory committees who had least sense of the importance of prevention, were not physicians with responsibility for people suffering from disease, and often were researchers fascinated by more and more research.
In the late 1970s, when involved in prevention of cardiovascular disease, I became vividly aware that refusal to agree that evidence is good enough, can have momentous and extremely serious consequences. At times of public health crisis, if no decisions are taken, this failure to judge, recommend – and then to act – may in effect cause the unnecessary suffering and deaths of hundreds of thousands if not millions of people, with all the associated bereavement of their families.
We all need to understand that by the nature of reality, evidence cannot ever be perfect or complete. The demand for more and more evidence may be right, but it may also be wrong. Much depends on the importance and urgency of the policy issues involved. A prime duty of the research science community is to decide when evidence from various sources is adequate – when, in the terms used by the WCRF reports (8-10), it justifies a judgement of 'convincing' or 'probable' causal connections and as such, a sound basis for action.
Who blocks public health action?
During the 30 years that I have been engaged with UN reports, it became generally accepted in the policy-making world that the standards for evidence should become much more rigorous, and that the quality and quantity of evidence should also increase. This sounds right and proper – and more on this below.
But during this period, all of us who were most deeply involved, including officials from government and the UN, were very impressed by the fact that the points about rigour, quantity and quality made in committee, were made most emphatically by researchers who were locked in to continuous series of clinical trials. We then slowly began to realise that if some interested parties were anxious that no policy should be developed on a topic such as saturated fat, salt or sugar, then an effective way to block any judgement was to highlight or exaggerate the statistical and other technical deficiencies in all the studies. Indeed, sometimes very rigorous, preferably statistically erudite, experts were commissioned to emphasise all possible objections, and such a spoiling tactic was then portrayed as cutting-edge science at its best.
I discovered this when working as a member of the UK government advisory committee on cardiovascular disease whose report was published in 1984 (11). We recommended limiting trans fats on biochemical and metabolic grounds to reduce the epidemic of heart disease in the UK. But then the milk and fat industries persuaded the member of the committee with most technical knowledge, to cast doubt on his own evidence!
Demand for more and more rigour, quantity and quality does not come only from research scientists. In 1990 the US government, the sugar industry, and member states whose economies at that time depended on sugar production, all claimed that the evidence judged by the independent scientists advising the UN for the WHO 797 report (6) to be an adequate basis for recommendations and thus public policies – on sugar and sugary products, for example – was actually grossly inadequate. This was repeated in 2003 with the WHO 916 report(7) when some US politicians, even up to the level of the President's Cabinet, pushed hard to get US funding of the World Health Organization stopped unless WHO and its advisors toed their line. A special meeting of agriculture ministers from all round the world also demanded that the Food and Agriculture Organization of the UN reject the 916 report on the grounds that it was both scientifically invalid and economically disastrous. This demand was based on a special report commissioned and paid for by the sugar industry, slanted to exaggerate threats from curtailed sugar production in the impoverished sugar-producing countries.
Vested interests
Most of all within the US government it is now almost impossible to make any judgement or recommendation at all, ever, on nutrition and health, alcohol and health, obesity, climate change, or many other important and urgent public health issues. There is also rapid interchange of staff between the higher ranks of the US government and those industries with a keen vested interest in influence on and control of public policy decisions and actions, particularly those that have a big impact on the money markets, commodity trading, agribusiness support systems, and health care. As an example, lobbyists for the tobacco industry, which has always insisted that the evidence on smoking and health falls far short of proof, become civil servants laying down the law on strength and quality of evidence! (12,13).
The intensity of lobbying in Washington, aimed at giving a freer hand to those industries whose policies and practices harm public health, is intense. It involves huge amounts of money for example donated to political campaigns. I have been told by influential policy-makers working for the main US political parties, that policies that would have the effect of impeding industrial interests would never be promoted in Congress or the White House. These views are amplified by the dominance in the US of the doctrines of the evil of government involvement, and of the sanctity of individual freedom. Community responsibility is discounted. This also explains the limited understanding of public health in the US. In many respects international corporations now have more power than do national governments especially in poorer countries. .In most European countries now the same power of industry is evident.
You might think that particularly outrageous and scandalous practices, such as the aggressive advertising and promotion of fatty, sugary or salty energy-dense food products and sweetened soft drinks to children, would trigger action in the public interest (14). On the whole you would be wrong. True, some governments, in response to persistent expert and public pressure, are actively interested in such legislation and other action, as I have recently outlined (15). But in general, public health nutrition issues are left to the consumer's 'choice' and 'the market' – meaning, industry, including that part of industry whose products, consumed in typical quantities, are harmful to health.
Box 2
Infectious disease is different
Occasionally the need to protect the public does take precedence over industrial and other vested interests. So far only major crises such as the BSE (mad cow disease) catastrophe have triggered firm policies that when enacted protect public health. Such crises typically are matters of serious or deadly infectious diseases, not chronic diseases.
I know about this from the inside. In 1998, Europe's chief veterinary officers as well as the affected industry, formally pressed for the scientific steering committee of the European Commission, the top expert advisory body dealing with any major health problem in Europe, to be sacked. They wanted the possible dangers of BSE spreading into the human population to be played down. I was one of the eight independent members of the committee, and was heavily involved in drafting the EC reports on BSE. We imposed for the first time stringent and disruptive controls on agricultural practices, despite the then very limited knowledge about the disease process or the infectivity of humans with prions – the proposed mysterious infective protein agent. We survived only because the European Parliament threatened to sack the President of Europe and his Commissioners if they did not deal rapidly with the BSE crisis.
So the Commission could not sack us without being sacked themselves. We were helped by the fact that our early predictions kept coming true, even though we were proposing policy on the basis of the very incomplete evidence then available – complex animal experiments, and fairly crude modelling of potential consequences to humans across Europe. We were doing the best that could be done in the circumstances, and were very well aware that worst-case scenarios for the spread of this deadly disease for which there is no treatment, were very grave indeed. Ironically our proposed solutions, when enacted, saved the European beef and milk industry billions of euros!
Evidence of what and for what
Now it is time for some explanation, of what I see as a curious confluence of interests between governments that avoid regulation, industries that hate regulation, and influential figures in the research science community.
Figure 1 below is a 'pyramid' designed to promote 'sound science'. It is a display of what is now a commonly agreed and promoted hierarchy of different types and levels of evidence designed to assess the health of populations. There are variations in the literature, and in guides to 'sound science' issued in particular in the US. In this pyramid, unlike the old US food guide pyramid, the 'best' is not at the base of the pyramid but at its apex. The general governing idea, agreed in more or less this form in the US, and increasingly within the UN system (see below), is that most types of research that have been regarded as valuable, in themselves or in combination with other types of evidence, actually have little or no value.
The pyramid here has a rigid epidemiological bias. It omits human metabolic and physiological studies in human health and disease. It also demotes or even dismisses epidemiological studies below the 'Cohort Studies' orange band, which should be essential guides to judgements, recommendations and actions, as I will set out later.
Figure 1
'Sound science' hierarchy of types of evidence claimed
as most relevant to public policy judgements and actions

Note. A more complete pyramid would insert Metabolic and Physiologic Studies, perhaps below Case Series, and Ecological (or Correlation including Migrant Studies), above Case Series. The inclusion of 'Editorials, Opinions' is obviously problematic – it depends on what evidence these are based on!
According to this hierarchy, studies involving test tube cultures or laboratory animals are no good as a basis for deciding whether any food or nutrient affects health, either in themselves or as contributory evidence, no matter how many such experiments are conducted. The inclusion of 'ideas, editorials, opinions' in the pyramid is rather strange. If meant to downgrade notions merely based on unverified 'received wisdom' or prejudice, this is sensible. 'Eminence-based' decision-making in clinical practice or in public health is indeed dubious practice. Experts have a duty to explain their judgements. But if this downgrading implies that whole expert reports whose judgements themselves are based on a great array of different forms of evidence (6,7) are of little or no value, we will never get anywhere!
A good example of why this and similar pyramids of hierarchy are foolish, is their apparent contempt for case reports, and series of such reports, of the type that may be communicated to regulatory authorities by physicians who notice that a surprising number of their patients are suffering or dying after taking some medicine. Thus alerted, the authorities limit the drug's use, demand rigorous rapid scrutiny of the drug's effects, or take the drug off the market.
By analogy, many physicians are horrified by the recent sharp rise in the numbers of obese children who now are consuming extraordinary quantities of fast food and soft drinks. More, they are scandalised. Given the grave outcomes of childhood obesity, in the UK the Academy of Medical Colleges is as I write, demanding immediate government action (16). Vested interests will demand careful controlled trials of childhood obesity prevention. Fortunately, influential health professional and civil society organisations are now combining, not only to demand action, but also to initiate action (17). A leader in this field is Boyd Swinburn, who with Shiriki Kumanyika is now co-chair of the International Obesity Task Force, and with her writes about the need for policy action in this month's WN. Nobody would seriously propose policy solely on the evidence of case reports. But clearly they are contributory evidence. They often are where public health reform starts (18).
A type of evidence that is absent from this pyramid (although it appears in others, near the bottom) is from ecological studies, also known as correlation studies. These observe similarities and differences between various populations. They include migrant studies. Thus, as an example, my colleague Larry Kolonel with colleagues has shown that there are impressive, consistent correlations between changes in the diets of ethnically Japanese people when they move from Japan to Hawai'i, and rates of stomach cancer (which fall) and of breast and colon cancer (which rise) (8). A classic example of the value of cross country studies is the Seven Countries Study of Ancel Keys and his colleagues which, supported by meticulous metabolic studies, has had a profound influence on policy-making (19).
Again, nobody would say that such evidence was in itself conclusive. The key factor might be something other than diet. But to discard such powerful suggestive evidence would be lunacy. This however is what the US pyramidologists, who include some epidemiologists, want to do, and their doctrines have now become internationally pervasive.
Moving up the pyramid, case-control studies are also now regarded as poor evidence at least in the cancer field. As applied to nutrition and health, these match cohorts of people with different characteristics (say, some with a specific cancer, others free of cancer) and identify differences in diet. These are better evidence than correlation studies, because they can control for potentially confounding factors, such as smoking, physical activity, and so on. To repeat, they are contributory evidence. Indeed, if the findings of case-control, ecological and migrant studies, say, contradicted the findings of the randomised controlled trials (RCTs) at the top of the pyramid (and I am coming to these), this would – or should – cast doubt on the results of the
But devoted followers of the US system, often interactive with conflicted industry, or from such industry, call all types of evidence as shown in the pyramid below cohort studies, 'junk science'. This includes almost all research of the types that funding bodies of lower-income countries can afford.
For a while cohort studies, shown in the brown band near the top of the pyramid, reigned supreme as 'sound science'. When prospective these are usually carried out over long periods of time, and in order to achieve statistical significance typically involve many thousands of people. They are very expensive, unless their analyses are based on postal questionnaires of self-assessed dietary intake and health outcomes. These have well-known drawbacks (20), which can however be validated by the use of biomarkers (21) as where possible is the policy of the European Prospective Investigation into Cancer and Nutrition (EPIC). The value of EPIC is increased by the wide ranges of dietary intakes from across Europe (22,23). Enthusiastic advocates of prospective trials overlook or discount the fact that many nutritional pathways in the body vary between individuals for genetic or epigenetic reasons. When prospective trials are used within populations – such as in the US – where ranges in dietary intake are modest, their results may have little meaning.
Evidence: the crock of gold
The one and only sensible approach in our field, is to respect and take into account all types of relevant evidence. Up to a point, this 'portfolio' approach was adopted in the 2007 WCRF report (9), and less systematically although perhaps more thoughtfully in earlier reports with which I was associated (6,8).
But among nutritional epidemiologists, who these days tend to call the shots in the human biological sciences, the hunt has been on for the ultimate 'knock-out' type of study that would discount all others. This would satisfy their desire for epidemiology to become a 'hard' science, worth funding with hundreds of millions of dollars, the kind of money that goes to researchers into the sub-cellular origins of disease. Hence the incessant demand for the randomised controlled trial (RCT), the red band in the pyramid, also known as the 'Cochrane' approach (see Box 3, below). RCTs are also claimed to be the 'gold standard' in epidemiology.
Randomised controlled double-blind studies are as close as experiments on humans can get to experiments on animals. They are crucial in examination of the safety of drugs. Volunteers are divided into groups. If the question is relatively simple, two groups are chosen. One is given the drug and the other is given an inert 'placebo' substance. Neither they nor the investigators know which is which (hence double-blind). After an agreed time the wraps come off and the results are studied. If the results are dramatic – if say, a lot of the subjects become very ill or die unexpectedly – then the trial is stopped early by an 'un-blinded' data safety monitoring board.
When an RCT is feasible and appropriate its results, when clear, produce strong evidence. The problem though, is that RCTs only work well with drugs! They usually can't work with food, because by its nature food is made up of many substances. (They can work with synthetic versions of vitamins, which are quasi-drugs). They also can't be used when the ill-effects of any substance are known or strongly suspected, because the professional committees that adjudicate on ethical issues won't allow this. Plus only some types of trial on food can be done blind, or double-blind, and then only with extreme difficulty. (Thus, two matched canteens could prepare and serve meals with identical sensory quality but with different nutrient compositions, where neither the investigator nor the subjects knew which canteen in serving which types of food).
So very curiously, a type of evidence regarded as a 'gold standard' in nutritional epidemiology is, as a basis for results and therefore recommendations and policy in our field, close to useless! Even when RCTs generate data, they cannot address whole foods or dietary patterns, let alone what drives food systems and supplies and what populations eat and drink, and why.
And so we come to the tip of the pyramid: systematic reviews and meta-analyses. In theory these should provide powerful evidence. But reviews and analyses of what? Reliance on systematic reviews and meta-analyses of cohort studies, as commonly specified now, is fraught with problems. Meta-analyses of cohort studies or of intervention trials depend on an accumulation of very expensive completed studies. For this reason, most evidence now counted as strong or 'sound' comes from studies carried out in and for high-income countries. Also, to ensure uniform results, systematic reviews and meta-analyses depend on pre-judged criteria to exclude studies that 'don't fit' and are judged as sub-standard. This pre-selection may in due course prove to be tendentious, arbitrary or mistaken.
And as you can see, all types of study regarded as even having a vestige of value as set out in the pyramid shown here, exclude any consideration of biological plausibility. As a physician this offends me. It also offends my common sense. But the idea that some things stand to reason is not allowed, along with all other ideas put down in the pink strip. You may be beginning to get the feeling that the only judgements admitted as a basis for public policies, are those generated by a quasi-mathematical process designed to evade judgement.
Box 3
Archie Cochrane and the Cochrane Collaboration
Archie Cochrane (1909-1988) a wonderful epidemiologist and public health expert, used to visit the Medical Research Council Epidemiological Research Unit (ERU) in Jamaica in the 1960s when I was there, and regaled Jean my wife and me with his life story and views. I met him because we lived in the special ERU apartments even though I worked for the Tropical Metabolism Research Unit dealing with complex studies in malnourished children.
Archie gave us vivid accounts of the time he was an ambulance driver in the Spanish Civil War, and a medical officer of health in wartime prison camps. He also continued to blast the stupid ways of medical practice that still persisted in the early days of the UK National Health Service. Oddly enough I had told my boss, Max Rosenheim (later president of the Royal College of Physicians of London, and then a Lord) that I had quit the UK for the same reasons, seeing 'clinical opinion' of the type that reigned supreme in those days as little better than witchcraft.
So Archie and I got on extremely well. During his time working for the Medical Research Council in Wales, he became an authority on the miner's disease pneumoconiosis. Arising from this and other careful work on diseases caused by pollution and infection which ravaged working class populations, he championed randomised controlled trials (RCTs) (24). After his death, in his honour, research centres called Cochrane Collaborations were set up, to champion the RCT method.
RCTs are now used appropriately for trials of drugs, and also in study of the types of disease that Archie himself specialised in, where a relatively few key factors are involved. They are also used inappropriately, and to be frank stupidly, for studies of food, nutrition and disease.
Archie himself would be horrified now to discover the rigidities and limited thinking involved in some Cochrane Collaboration analyses. He would for sure have said that the definition of any question to be answered with a 'Cochrane' analysis, must take into account a wide range of knowledge, and not simply used as a crude recipe which often – and indeed usually, in our field – leads to misleading and even meaningless conclusions.
Evidence: the answer
So as somebody who has been working in the fields of medicine, nutrition, and public health for half a century, mostly at a pretty high level, I do have some opinions about evidence (25). For a start, it is clear to me that any attempt to sweep away and discard most lines of evidence is wrong and indeed highly suspect.
Some types of laboratory study have limited value but are necessary in a toxicological context. I certainly consider that studies designed to establish biological plausibility, especially when carried out by investigators who understand how human and other living systems work, are vital.
However, I am decidedly sceptical about many epidemiological studies whose methods conform to current concepts of validity, but which are often not methodologically sophisticated, and lead to false negative findings or else do not make biological sense. And I am implacably hostile to the current trend to demand ever more evidence, with randomised controlled trials as the hallmark of all future needs. This overlooks the known benefit of major public health initiatives undertaken in the past that followed careful intelligent scrutiny of different types of evidence, with recognition that when public health action is taken, its effects must be monitored and when necessary the actions reinforced or revised.
We all need first, to recognise that evidence by its nature is never complete. Second, that in matters of public health and the public interest, those involved in policy-making have a duty to decide when evidence is good enough. Third, that nothing beats human intelligence, and this means we continue to need independent specialists with a broad understanding to come and reason together, and to make the best judgements they can. If this makes me sound old-fashioned, so be it.
And now I come to the immediate reason for this commentary.
Evidence of value
With all this in the back of my mind, in April (this month, as I write) I attended in London a special meeting of NICE. This is the National Institute for Health and Clinical Excellence. The specific purpose of the meeting was to consider whether the ranges of body mass indices (BMIs) that are applied generally, whereby BMIs between 25 and 29.9 are classed as 'overweight' and 30 and above as 'obese', are the right ranges in the UK for so-called ethnic minorities – the Asian and Black communities.
Bear with me please, while I explain a bit about NICE, which is renowned for its independent and carefully documented ways of considering evidence. Its work is very relevant to ours, and now more so than ever. It serves the English and Welsh national health services. It is best-known for its work evaluating the value of drugs. Since its foundation in 1999, I have been impressed and influenced by the clinical discussions of NICE about cost-effectiveness analyses. NICE has gained a terrific reputation for its routine evaluation of different drugs for use in the UK. The evaluation is not done in terms of safety and whether a drug should be allowed on the market. This is governed by CHMP (the Committee for Medicinal Products for Human Use), the European counterpart to the US FDA, but which deals only with health, not food. So if a drug is cleared for clinical use by CHMP it can legally be prescribed, unless a national regulatory body of a European country disagrees and restricts its use in some way. This process protects doctors from accusations of improper practice as long as they follow the guidelines for its proper use.
In the UK, however, there is a further hurdle, which is whether any drug is of sufficient value to warrant its use within the National Health Service, where almost all the cost of drugs is paid with public money. Thus, some drugs identified as safe and also effective may be withheld if they are very expensive, especially if they are of only limited value. NICE has developed a very careful process of impartial analysis using health economists (usually from York University) which works out the cost per year of the drug for every year of quality life estimated to be gained. This is not just days or weeks of longer life, specified as Life Years Gained, or LYG, but how well any given drug deals with disability and quality of life associated with the disease. These costs are set out as costs per Quality Adjusted Life Years, or QUALYs. This distinction between living longer and being free from disabilities is obviously important, and in principle is easy to understand, although how economists are able to work out in $US or £ the value of all sorts of different qualities of life is a bit of a mystery to me.
Over the years, NICE has rejected several drugs on the grounds that they are not cost-effective and so do not warrant the use of taxpayers' money for treating anybody who in their physician's view needs the drug. We read about such decisions occasionally as being not merely hard-headed, but as death-sentences – but clearly there must come a point within any country, even at times of general prosperity, when a drug is too expensive.
I first engaged with NICE because of my work as a physician with a lot of experience of ethical and effective clinical practice, and with a special interest in obesity. I helped design the first major trial of Orlistat, the first drug designed to treat obesity. Unknown to me at the time, the outcome of this trial was crucial to the submission that Roche, its manufacturer, made to CHMP and the FDA. Originally I had advised Roche not to develop the drug, because it only blocked one-third of fat absorption.
What then astonished me – and many others – was that NICE analyses showed that Orlistat was proving highly cost-effective, because it cost about £10,433 per QUALY gained, compared with a cost of £66,926 for each life year gained (26). This seemed to me to be hugely expensive. But then I learned that drugs in the UK were considered expensive if on a QUALY basis they cost £60,000 to £75,000, and that Orlistat was less expensive than many standard treatments. Indeed the cost was estimated to be only about £2,850 per life year gained (LYG) for obese diabetic patients with hypertension and hypercholesterolemia (27). This cost was one of the lowest for any medical therapy – about the same as immunisation with a hepatitis vaccine.
This made me think harder about the issue of cost-effectiveness in other areas of public health. As NICE progressed its work, I gradually came to realise that this careful and rational approach to cost-effectiveness could be applied not only to drugs, but to any area of public health – including food and nutrition. Even before NICE was set up, with colleagues I had a stab at this, in the public policy chapter of the first WCRF report (8). I felt then, and am sure now, that such calculations, of the costs of obesity and chronic diseases, and of the cost-benefits of preventing diseases, are essential if we are ever going to persuade politicians of the need for prevention.
Evidence for public health
That's some context. Now, back to the April NICE meeting. Given what I knew up to then about NICE methods, I was assuming that we would be asked to think in economic terms in deciding suitable BMI ranges and cut-offs for different ethnic groups.
Not at all! We were told that we were now in the public health section of NICE, and that the standards and methods that apply to drug policy – and as I have shown above, that are now being misused in formulation of nutrition policy – do not apply to public health policy. Further, we were told that in public health issues, it made no sense to insist on evidence from intervention trials – those on top of the Figure 1 pyramid above – before determining policy.
This news astounded me. As I have said above, most expert consultations, including even several of those now convened by WHO, insist on a process of 'systematising' how we think. What this means in practice, is that even nutrition policy making is rapidly moving to a process based on decision making for drugs. Members of committees are seemingly no longer permitted to cite coherent, sensible types of evidence consistent with knowledge of the biological mechanisms of disease, or coherent human feeding and metabolic experiments. It seems that this is now against the rules.
Instead, committees are increasingly required to sit in judgement on the pre-packaged results of 'systematic reviews' of the epidemiological evidence – at the top of the pyramid – specially pre-commissioned from separate teams who crunch numbers with sophisticated statistical techniques according to pre-specified rules. This cumbersome process can reduce expert committees to tickers of boxes. It is difficult to resist the conclusion that the experts are invited on to these committees in order to lend their reputations to a process that blocks them from using their knowledge and intelligence, and instead asks them to rubber stamp the often crude conclusions presented by the systematic reviewers.
Worse yet, there has been a demand recently that policy experts accept that public health interventions, including those concerned with food and nutrition, must be supported by 'proof' from intervention trials before recommendations can be made, let alone actions taken. This approach is happily accepted by some nutritional epidemiologists who get grants for interventions that they can call 'trials'. Leaders of epidemiological teams who specialise in systematic reviews are understandably, very supportive of policies that give their own work more importance. So those of us who seek to improve public policies are now confronted by scientists who should be concerned with public health, but who in practice are acquiescing in a reversal of all the lessons learned from nutritional policy-making over the years.
Judgements, recommendations, policies and actions in many areas of our field are needed now. These are urgent and crucial at this time when rates of obesity, diabetes and diet-related cancers and other serious diseases are sharply increasing, particularly among the most vulnerable populations.
Wider dimensions
We have allies. At the UK National Institute for Health and Clinical Excellence the tide has turned. You can imagine how delighted I was to hear what NICE has concluded, on issues to do with public health. All the more so, when it was explained to us just how much careful debate had preceded publication of their report explaining and justifying the methods used (5).
This is a marvellous document. It specifies that 'in public health, social scientific as well as clinical and epidemiological evidence is used to examine outcomes, context, process and implementation (as well as barriers to and facilitators for intervention)'. While they say there is as yet 'little academic consensus on how best to synthesise these different approaches and there is still less agreement about how to use these different disciplines to develop guidance', they show how foolish it is in public health to rely only on intervention trials as the basis for public health policy making
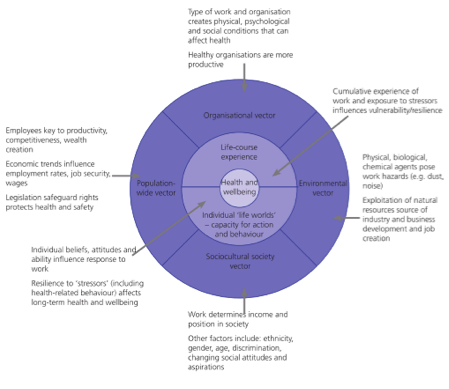
The report takes into account other social and also political factors that influence public policy decision-making. As well as evidence from the social sciences, the report insists on the need to take evidence from the people who are most affected. It also specifies the need to take into account, evidence of what is most likely to work best in any social setting. The report includes some superb graphic illustrations of their thinking. Take Figure 2 below, which projects their analysis of mental well-being at work.
Figure 2
A conceptual framework for promoting
mental well-being at work
Suddenly, mathematics is given its proper place, as a tool to be used within a human context. This need to consider context-specific evidence is projected by another one of their superb graphics, shown here as Figure 3:
Figure 3
Evidence: context-sensitive complements context-free

Evidence for us
Within the Association we agree that nutrition has social, economic and environmental as well as biological and behavioural dimensions. We also agree that disease, health and well-being have basic and underlying as well as immediate causes. What I have set out here strengthens the case to define public health nutrition as a branch of public health. I now propose that the NICE report becomes the key resource for new Association working groups. On this, please see Box 1 at the beginning of this commentary. NICE has challenged us to rise to its standard in our work. Let us accept the challenge.
What then, about the issue of standards for assessing overweight and obesity among ethnic groups in the UK? I guess this is for another time!
It is time to speak out, and act
Encouraged by the work now being done by NICE, and also by the US National Institute of Medicine and other leading expert organisations, I believe that we in the public health and nutrition professions must now speak out collectively. Action in the public interest is now being thwarted on practically all public health issues, including those that are most menacing.
UN officials are under intense pressure to be ever more scientifically rigorous. This sounds right, but can have the effect of narrowing the basis of their decision making. At the same time, UN agencies are being pushed into so-called 'public-private partnerships' where the private 'partners' are international corporations whose interests conflict with those of public health and where the profit motive is paramount.
Our task now as professionals in the field, is to help public-spirited UN officials. We need to urge them to keep conflicted interests out of policy-making and to engage such interests only in the practicalities of implementation. We also need to expose and denounce the fact that more and more national governments are apparently now only interested in policies and actions that are backed – or initiated – by the corporations responsible for the energy-dense, fatty, sugary or salty highly processed products that are a main cause of the crisis.
We also have to guard against self- serving scientists demanding ever more research money for their own work, as well as the commercial interests that also sabotage policy development. If these forces are not countered effectively, then policies and actions that really could improve population nutrition and public health will continue to be thwarted.
The counter-forces need to include us who are engaged in public health knowledge and policy. We have to be as erudite, robust, and respectful of evidence as the most rigorous of those blocking progress. We also have to change the dimensions of debate, and weigh the potentially major costs of doing nothing, with inevitably incomplete data and any possible chance of doing harm.
Our challenge is to make sure that policies are agreed at the right speed. It is also to return policy-making to an appropriate, intelligent analysis of all relevant available evidence.
References
- Institute of Medicine. Bridging the Evidence Gap in Obesity Prevention. A Framework to Inform Decision Making. Washington, D.C.: The National Academies Press, 2010
- Kumanyika S, Economos C. Prevention of obesity. Finding the best evidence. [Commentary] World Nutrition, June 2011, 2, 6: 272-299. Obtainable at www.wphna.org
- Swinburn B, Gill T, Kumanyika S. Obesity prevention: a proposed framework for translating evidence into action. Obesity Reviews, 2005,6:23-33.
- Institute of Medicine. Progress in Preventing Childhood Obesity. Washington, D.C.: National Academies Press, 2007.
- National Institute for Health and Clinical Excellence. Methods for the Development of NICE Public Health Guidance. Second edition, London: NICE, 2009. See: http://www.nice.org.uk/aboutnice/howwework/developingnicepublichealthguidance
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases. Report of a WHO study group. Technical report series 797. Geneva: WHO, 1990.
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases. Report of a joint WHO.FAO expert consultation. Technical report series 916. Geneva: WHO, 2003
- World Cancer Research Fund/ American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington DC: AICR, 1997.
- World Cancer Research Fund/ American Institute for Cancer Research. Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Washington DC: AICR, 2007.
- World Cancer Research Fund/ American Institute for Cancer Research. Policy and Action for Cancer Prevention. Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Washington DC: AICR, 2009.
- Department of Health and Social Security. Diet and Cardiovascular Disease. Committee on Medical Aspects of Food Policy. Report of the panel on diet in relation to cardiovascular disease. Report 28. London: HMSO, 1984
- Federal Focus. Principles for Evaluating Epidemiologic Data in Regulatory Risk Assessment (The 'London Principles'). Agreed in 1995. Obtainable at: http://www.fedfocus.org/ Accessed 13 April 2012.
- Mooney C. Wine, jazz and data quality. [Chapter 8]. In: The Republican War On Science. New York: Basic Books, 2005.
- Swinburn B, Sachs G, Lobstein T, Rigby N, Baur L, Brownell K, Gill T, Seidell J, Kumanyika S. The 'Sydney Principles' for reducing the commercial promotion of foods and beverages to children. International Obesity Task Force Working Group on Marketing to Children. Public Health Nutrition 2008, 11, 9, 881-886.
- James WPT. As I see it. Website of the World Public Health Nutrition Association, April 2012. Obtainable at: www.wphna.org.
- Campbell D, Boffey D. Doctors turn on No 10 over failure to curb obesity surge. The Guardian, 14 April 2012.
- Gortmaker SL, Swinburn BA, Levy D, Carter R, Mabry PL, Finegood DT, Huang T, Marsh T, Moodie ML. Changing the future of obesity: science, policy, and action. The Lancet 2011; 378, 9793, 838-847
- Hempel S. The Medical Detective. John Snow and the Mystery of Cholera. London: Granta Books, 2007.
- Keys A. (Ed). Seven Countries. A Multivariate Analysis of Death and Coronary Heart Disease. Cambridge MA: Harvard University Press, 1980.
- Freedman LS, Schatzkin A, Thiebaut AC, Potischman N, Subar AF, Thompson FE, Kipnis V. Abandon neither the food frequency questionnaire nor the dietary fat-breast cancer hypothesis. Cancer Epid Biomarkers Prev2007; 16 (1):181-182.
- Freedman LS, Kipnis V, Schatzkin A, Tasevska N, Potischman N. Can we use biomarkers in combination with self-reports to strengthen the analysis of nutritional epidemiologic studies? Epidemiologic Perspectives and Innovations 2010, 7, 2.
- Riboli E. The European Prospective Investigation into Cancer and Nutrition (EPIC): plans and progress. Journal of Nutrition 2001, 131(1)
- Chajès V, Biessy C, Byrnes G, Deharveng G, Saadatian-Elahi M, Jenab M, et al..Ecological-level associations between highly processed food intakes and plasma phospholipid elaidic acid concentrations: results from a cross- sectional study within the European Prospective Investigation into Cancer and Nutrition (EPIC). Nutrition and Cancer 2011; 63: 1235-1250.
- Cochrane AL. Effectiveness and Efficiency: Random Reflections on Health Services . Second edition. London: Nuffield Provincial Hospitals Trust, 1989. (First published 1972).
- James WPT. UN Summit on non-communicable diseases. Up to the Summit: Inglorious paths.[Commentary] World Nutrition, September 2011, 2, 8: 352-399. Obtainable at www.wphna.org.
- See: http://www.nice.org.uk/Technical appraisal guidance no 22
- Lamotte M, Annemans L, Lefever A, Nechelput M, Masure J. A health economic model to assess the long-term effects and cost-effectiveness of Orlistat in obese type 2 diabetic patients. Diabetes Care2002, 25, 303-308.
Acknowledgement and request
Readers are invited please to respond. Please use the response facility below. Readers may make use of the material in this commentary, provided acknowledgement is given to the authors and the Association, and WN is cited.
Please cite as: : James WPT. Rio2012. What next. Coming to judgement [Commentary] World Nutrition, May 2012, 3, 5: 179-202. Obtainable at www.wphna.org
The opinions expressed in all contributions to the website of the World Public Health Nutrition Association (the Association) including its journal World Nutrition, are those of their authors. They should not be taken to be the view or policy of the Association, or of any of its affiliated or associated bodies, unless this is explicitly stated.
My thanks to Geoffrey Cannon, who provided valuable background information and proposed my expanding an originally quite short piece. He also supplied the illuminating pyramid shown as Figure 1. WN commentaries are subject to internal review by members of the editorial team.