World Nutrition
Volume 2, Number 9, October 2011
Journal of the World Public Health Nutrition Association
Published monthly at www.wphna.org
The Association is an affiliated body of the International Union of Nutritional Sciences For membership and for other contributions, news, columns and services, go to: www.wphna.org
Commentary: Breastfeeding
The need for law and regulation
to protect the health of babies

George Kent
Department of Political Science, University of Hawai'i
Email: kent@hawaii.edu
Click here for member's profile
(i) Promote, protect and support breastfeeding, including exclusive breastfeeding, for about six months from birth, as appropriate, as breastfeeding reduces susceptibility to infections and the risk of undernutrition, promotes infant and young children's growth and development, and helps to reduce the risk of developing conditions such as obesity and non-communicable diseases later in life; and, in this regard, strengthen the implementation of the International Code of Marketing of Breast Milk Substitutes and subsequent relevant World Health Assembly resolutions.
UN High-Level Meeting on NCDs. Political Declaration, Clause 43 (i)

Even footballers thrive on breastmilk. A supporter of England and also of Brazil in the 2006 World Cup, just over two years old, enjoying his lunch
Breastfeeding is universally agreed to be vital for the health and well-being of infants and young children. But very often, public and private policies and practices, including those of governments, institutions and employers, stack the odds against breastfeeding and in favour of formula feeds. Following the recommendation shown above, adopted in September by the UN High-Level Meeting on the prevention and control of non-communicable diseases, it is time to give more and stronger support to breastfeeding, and correspondingly further to restrict formula feeding. This implies strong regulation. It often implies the use of law – statutory regulations, that protect the health and freedoms of children and their mothers, families and communities.
Breastfeeding is almost always better for children's well-being than feeding with infant formula. Nevertheless, many people, especially in high-income countries, believe that infant formula is both safe and nutritionally adequate. People often assume that their governments would not allow the product to be sold if it were not good for their children. These are errors.
The prevailing standards for the composition of infant formula say that infant formula must include a list of specified ingredients. This approach fails to appreciate the complexity and the full functionality of breastmilk and breastfeeding. Feeding methods for infants and young children need to be safe and nutritionally fully adequate, and also need to contribute to the child's sense of security and well-being and general mental and emotional health.
Breastfeeding is now known to protect against breast cancer in the mother and probably against obesity in the child (1), as well as against nutritional deficiency and infectious diseases. The implications of feeding practices on children, their mothers and families, and on the formula manufacturers, therefore need radical review. What health benefits outweigh wealth gained by industry? To what extent can commercial success, which includes investment and employment benefits, justify proven substantial risk to the health and life of children? And how should such judgements, and decisions flowing from them, be best made?
In my view, the primary interest of regulators of food for infants and young children should be the well-being of the children, at the time and also throughout their lives. Other interests may also be served, but not in ways that put children at significant risk. But some of the current standards and practices approved and applied by governments and regulatory agencies do endanger infants and young children. Stronger regulation is therefore needed to ensure that the nutrition of infants and young children is always adequate and as often as possible, optimal. There is a need for the use of law, to ensure that children, and also their mothers and families, are protected.
Breastfeeding compared with formula
The choice of feeding methods has consequences for the child and also for the mother. In the US the government's Office on Women's Health identifies the major benefits of breastfeeding in terms of nutrition and growth benefits, and also enhanced immune systems and resistance to infection. It further describes the health benefits for the mother (2). The American Dietetic Association provides similar information on the health benefits of breastfeeding for both the child and the mother (3). It is best not to speak of the benefits of breastfeeding, so much as to view breastfeeding as the standard against which infant formula and other forms of replacement feeding should be assessed (4,5).
Breastfeeding has significant economic consequences. It yields savings because of the elimination of the cost of formula and also because of averted health care costs. A US government study estimated that every year 'A minimum of $3.6 billion would be saved if breastfeeding were increased from current levels . . . to those recommended by the US Surgeon General, … not counting the savings of the cost of formula. This figure is likely an underestimation of the averted health care costs because it represents cost savings from the treatment of only three childhood illnesses' (6).
The savings would be even greater if breastfeeding were to follow United Nations recommendations. The World Health Organization and the UN Children's Fund (UNICEF) recommend that all infants should be exclusively breastfed for six months, and that breastfeeding should be continued, with appropriate complementary feeding, for up to two years and beyond (7). A study in Pediatrics estimated that the savings in the US alone resulting from improved breastfeeding practices would be about $13 billion per year (8).
When not to breastfeed
There are few conditions under which children should not be breastfed by their biological mothers. It is reliably agreed that here are no nutritional contraindications to the breastfeeding of infants except for those with special health needs such as galactosemia or phenylketonuria. Breastfeeding by the mother is not recommended when the mother has certain infectious diseases, has taken certain medications or street drugs (9), or has certain environmental contaminants in her breastmilk. The general recommendation is that breastmilk should not be withheld from any infant unless absolutely necessary (10). The American Academy of Pediatrics recommends that: 'Pediatricians and other health care professionals should recommend human milk for all infants in whom breastfeeding is not specifically contraindicated' (11).
Premature infants and infants with disabilities might not be able to breast feed, but nevertheless they would benefit from breast milk, whether from their own mothers or from donors. For infants who are unable to breastfeed directly from their mothers, the best alternatives are using the mother's own expressed milk, or donor milk from another woman, which may need to be pasteurised or heat-treated.
Hazards of formula
Many studies have demonstrated the linkages between infant feeding patterns and morbidity and mortality. These can be dramatic. For example, a study in Brazil showed that the risk of death for infants who did not receive any breastmilk was fourteen times higher (12). On a population basis, use of infant formula always increases the risk of child illness and death. (4, 8, 10, 13-22). It is estimated that if every infant was exclusively breastfed from birth for six months worldwide, more than a million lives could be saved each year (23).
An early study in the US found that bottle-fed babies were six times more likely to die than breastfed ones. Additional studies showed the same six-fold increase in risk for relatively impoverished families, and a four-fold increase in risk for relatively wealthy families (24). With time, the quality of formula improved, and it is now usually subject to regulation (25). But despite this, formula feeding continues to cause substantial numbers of excess infant deaths. According to a US study published in 2004, the risk of post-neonatal (29-365 days of age) mortality is now over 25 per cent higher among infants who are never breastfed compared to infants who are ever breastfed. On this basis, about 720 infant deaths in the US would be averted each year if all infants were breastfed (13).
This is an underestimate. First, the study excluded neonatal deaths (0-28 days). Second, 'because exposure to breastfeeding was categorised based on the infant ever being breastfed, the estimate is based on a mixture of breastfeeding exposure levels, including many who were breastfed for a very brief period… the estimate of 720 lives saved is likely to be an underestimate compared to the additional effect of continued breastfeeding' (26).Third, if deaths beyond the first year were included, the estimate for the number of deaths associated with not breastfeeding would be higher.
Apart from the higher mortality, there are many children's illnesses that increase as a result of not breastfeeding. In both high-income and low-income countries, the consequences of not breastfeeding are more likely to show up as increasing likelihood of illness rather than death.
Practically every study that compares the health consequences of breastfeeding with the health consequences of using formula in a population, shows that using formula leads to worse health consequences. The harm may last into adulthood (1,14, 19, 21). Studies that assess only short-term impacts underestimate the total harm that results from formula feeding.
There is far less morbidity and mortality resulting from using formula in high-income countries than in low-income countries, one reason being that in high-income countries water is usually safe. But in any setting, compared with breastfeeding, formula feeding is risky. The only questions are, how risky, risk of what, and the implications.
Box 1
Formula safety standards
At the international level, non-binding guidelines regarding food safety are developed by the Codex Alimentarius Commission, established in 1963 by the Food and Agriculture Organization of the United Nations and the World Health Organization (27).
Codex Alimentarius
In 1976 Codex issued a Statement on Infant Feeding. It said, 'it is necessary to encourage breastfeeding by all possible means in order to prevent that the decline in breastfeeding, which seems to be actually occurring, does not lead to artificial methods of infant feeding which could be inadequate or could have an adverse effect on the health of the infant' (28).
At this session the commission also adopted a Codex Standard for Infant Formula. This includes a list of required ingredients and names various required quality control measures. It was adopted in 1976. In 1983, Codex adopted amendments to the sections on Food Additives and Labelling. Further amendments have been adopted (29).
Codex has a Code of Ethics for International Trade in Food, adopted in 1979 and amended in 1985. The preamble's paragraph acknowledges the International Code of Marketing of Breast-milk Substitutes, an agreement adopted by the World Health Assembly in 1981 (30). Together with subsequent relevant WHA resolutions, the marketing code sets out guidelines for appropriate marketing of breastmilk substitutes (24, 31-32).
The US, the UK and Europe
In the US, the Code of Federal Regulations specifies infant formula quality control procedures. The rules have been summarised as follows: 'Infant formula, like no other food, is regulated by its own law, the Infant Formula Act of 1980 as amended in 1986. The act sets lower limits on 29 nutrients. . . . Manufacturers are required to follow "good manufacturing practice," but no requirement for sterility is specified. In fact, the FDA performs bacterial counts on infant formula, and up to 10 000 colony forming units per gram powder are acceptable. Powdered formula is not guaranteed nor required to be free of pathogenic organisms' (35).
Standards in the UK are similar. The regulations provide detailed specifications regarding the composition of infant formula. Unlike the US standards, they incorporate detailed regulations based on the WHO International Code of Marketing of Breast-milk Substitutes (31). The UK regulations now specify required ingredients (36) and the amounts of allowable pesticide residues (37).
The European Community has followed this general pattern of focusing on ingredients (38)l and says: 'infant formula is the only processed foodstuff which wholly satisfies the nutritional requirements of infants during the first four to six months of life'. It is not clear how they arrived at that conclusion.
The formula manufacturers take the position that they are not responsible if their product is not used as directed. They insist that, apart from a few exceptional contamination incidents, their products are safe, even if the methods of using them are often unsafe. Codex and most national regulatory agencies seem to have accepted this position.
How unsafe is formula?
Powdered infant formula is not a sterile product. It deteriorates over time. Therefore, the UN Codex Alimentarius standards for infant formula specify: 'The date of minimum durability (preceded by the words "best before") shall be declared by the day, month and year in uncoded numerical sequence, except that for products with a shelf-life of more than three months, the month and year will suffice' (29). See also Box 1.
Outdated formula
But apparently there are no regulations to prevent misuse of outdated or even defective infant formula. In most countries there are no laws against selling outdated formula, nor requirements as to what is to be done with outdated or recalled formula. Nor are there tracking systems in place. A strong system of regulation could prescribe methods of disposition, with requirements for careful record keeping of the chain of custody until the product is disposed of under prescribed rules.
The GRAS concept
The UN Codex Alimentarius food safety system is based on whether particular food items in themselves are safe to use. So are national systems. In the US the requirements for many foods, including infant formula, are set out in the Food, Drug and Cosmetic Act. Thus: 'All manufacturers of infant formula must begin with safe food ingredients, which are either generally recognized as safe (GRAS) or approved as food additives for use in infant formula' (39).
A food described as GRAS is one that is therefore classified as not in need of oversight. Most foods are routinely categorised as GRAS. Most infant formulas are based on either cow's milk or soya milk, both of which are categorised as GRAS. Thus, basic infant formula is assumed to be safe. On soya, see Box 2.
This is an unsound assumption. The GRAS concept makes some sense when assessing whether a food item is reasonably safe to include in a normal adult diet. It is wholly inadequate when that food item is the diet. Hamburgers are reasonably safe to eat when they are part of a diverse diet, but as shown in the Morgan Spurlock film, Super Size Me, hamburgers are in effect toxic when they constitute the bulk of, let alone the whole diet. Infant formula constitutes practically the entire diet, and also is consumed by highly vulnerable infants. Showing that something has been safe for adults does not mean that it is safe for infants.
Box 2
The case of soya-based formula
Soya-based formula illustrates the issue of safety. Soya milk is categorised as safe because historically soya has been used as an item in human diets in many forms with no major problems. The categorisation as safe, was carried over to its use in infant formula. This was despite the fact that there had been no prior experience with using soya milk as practically the entire diet, whether for adults or for infants.
There have been studies that assert that soya is safe to use in infant formula (40-41). One report acknowledges: 'Some child-advocacy groups claim that consuming soy-based formula could accelerate puberty and cause developmental and reproductive abnormalities and thyroid disorders later in life'. It then describes a major six-year USDA-funded study to assess the 'long-term health effects of soy infant formula' (42-43). It is not clear how a six-year study of young children could assess an inherently long-term health effect such as sexual dysfunction.
Many reports have raised unanswered questions about the safety of soya, both for the general population and for infants in particular (44-45). The American Academy of Pediatrics has given up its 1998 position and now, with few exceptions, recommends against soya formula (46).
It is irresponsible to simply assume that soya-based infant formula is known to be safe and does not require specific studies of its safety in actual use.
Genetically mofified soya
While soya has long been used in the human diet, genetically modified soya is new. Nevertheless, it has been categorised as safe. It is difficult to see how the term 'generally recognised as safe' can legitimately be used to describe a novel product. Much of the testing of genetically modified soya has been done by Monsanto (47). There has been little independent testing.
The use of genetically modified soya in infant formula is even newer than its use in adult diets. Genetically modified soya has been categorised as safe even when it is used as the basic component of infants' entire diet. Questions have been raised about the wisdom of simply assuming that genetically modified soya can safely be used as the basic component of infants' diets (48). Heinz and Wyeth have agreed not to use genetically modified soya in their infant formula (49-50). In Australia, Greenpeace led a campaign to remove soya-based formula from stores because it was not clearly labelled as using genetically modified ingredients (51).
Major formula companies such as Nestlé, Mead Johnson, and Ross (Abbott) use genetically modified soya in their infant formula and other infant food products (52). They defend it by saying it meets current safety standards. That does not justify the practice. It raises questions about the adequacy of the standards.
Curiously, in its critique of soya-based formula in 2008, the American Academy of Pediatrics did not discuss the distinctive issues that might arise when it is based on genetically modified soya (46).
Almost all soya now produced has been genetically modified, so most soya-based infant formula is likely to be based on genetically modified soya, even if not acknowledged. This may be why the International Dairy Foods Association no longer says, 'because GM ingredients offer no particular benefit over the traditional sources at the present time, IDFA members take all possible steps to ensure that ingredients used in baby foods are not derived from genetically modified crops'. This is no longer a practical recommendation! The Association no longer says, 'Where there is the potential for GM material to be present from, for example, soya or maize, companies source non-GM, identify preserved ingredients through carefully audited suppliers with independent testing' (53).
Variations in formula
In the US, the government's safety guidelines for infant formula as set out in 1985 said: 'A manufacturer must notify the FDA 90 days before the first processing of any infant formula for commercial or charitable distribution for human consumption that differs fundamentally in processing or in composition from any previous formulation produced by the manufacturer' (54).
This means government is concerned with assessing the effects on safety of any new ingredients proposed for infant formula. There is detailed guidance on how these incremental changes are to be assessed (55). The manufacturer must demonstrate the safety of changes in the product. But there is no requirement to demonstrate the safety of the baseline infant formula product. The concern is with reformulation, not with the basic formulation. The uncritical readiness to assume that new variants of infant formula are safe to use should be challenged. It exposes children to unnecessary risk.
How hazardous is formula?
Feeding with infant formula is less safe than breastfeeding. So should feeding with formula should be regarded as unsafe, with all this implies – or should imply?
Safety can be assessed with various kinds of indicators, such as counts of adverse events, or estimates of excess deaths. No matter what indicator is used to measure safety, there is always a question of how it should be interpreted. Where on the continuum should particular practices be judged to be sufficiently unsafe to be in need of public policy intervention, which may need the use of law? What standards should be used to make that judgment? What is significant risk?
It is commonly assumed that in high-income countries, formula feeding is a reasonable second-best choice. Many parents' experience tells them that using formula is indeed safe. Their neighbours feed their infants formula, and they seem okay. People tend to assume that if the government allows the product to be sold, it must be safe. The 'second best' position is that taken by the US Food and Drug Administration (56). For many, the choice of second-best is not a big issue. It sounds rather like choosing white rice over brown rice, even though brown rice is better for you.
There is a major difference, however. For most people, rice is just a part of a diverse diet. Whatever is missing from white rice or any other sub-optimal food may be provided in some other part of the diet. The risks of making a wrong choice are much higher when the diet is not diverse. White rice is safe to eat, but a diet of white rice alone is not.
In terms of its impact of child health, breastfeeding is best, and optimal breastfeeding means early initiation (in the first hour after birth), exclusive breastfeeding for six months and continuing breastfeeding for up to two years or more (7). Second best is not formula, it is expressed breastmilk from the biological mother. Third best is wet-nursing, meaning breastfeeding by a woman who is not the biological mother. Fourth best is milk from a human milk bank, where these exist. Formula comes in fifth. Of course in terms of considerations other than child health, such as convenience to the mother, formula might be ranked higher.
When should access to formula be limited?
In any country, reliable data are needed on the extent to which formula feeding causes additional child disease and death. Then agreed standards are needed on which judgements can be made, derived from these data, as to whether formula use is acceptable or not. How much added disease and death is acceptable? As stated above, a recent study, likely to be an underestimate, calculated that in the US there are about 911 excess infant deaths each year likely to be averted by even modest duration of breastfeeding (8). Is that a trivial number, or a reason for the use of statutory regulations to restrict the use of formula? What criteria should serve as the basis for public policy?
Rational risk management of infant formula could be guided by policy for other hazardous products. For example, when there are a small number of deaths associated with defective toys or contaminated foods, those products are recalled very quickly. Should infant formula be treated differently? Why is it that in the US the Consumer Product Safety Commission becomes alarmed when drop-side cribs cause 32 infant deaths over a ten-year period (57), but the FDA seems unconcerned by the estimated 911 deaths of infant every year? Suppose formula was categorised as a pharmaceutical product, or a dietary supplement. Would regulatory authorities then withdraw it from the market? One clear difference with the defective toys or cribs, is that these are not the norm. With formula, the hazard is intrinsic to the product when it is manufactured according to current standards. Another difference is the scale of the commercial value of formula.
Relative risks
The risks of formula in use are not distributed evenly within any population. Of course the risks tend to be much greater in low-income countries than in high-income countries. There are important variations within countries as well. It is in the US as a whole that mortality is over 25 per cent higher among infants who are never breastfed (13). However, impoverished families face higher risks when using formula than the wealthy, in all countries. In many countries impoverished communities have far lower breastfeeding rates.
One approach to standard setting would be as follows. The standard would say that if the best available scientific data indicate that children fed with infant formula show a consistently significant higher level of morbidity or mortality, compared with breastfed children in the same population, then, given the evidence of biological plausibility, this means the risk is significant. The implication would be that public policies and actions were needed. In such cases, the use of statutory regulation further to restrict the availability and use of formula must be a serious option for legislators.
Breastfeeding: Nourishing and nurturing
Benefits of breastfeeding go beyond the nutritional quality of breastmilk.
The nurturing of this child by his mother is evident here in these pictures
Infant formula is inferior to breastmilk. But the benefits of the breastfeeding process go beyond those delivered by breastmilk itself. The true comparison is between formula feeding and breastfeeding. As shown in Figure 1, the major underlying causes of child malnutrition are related not only to food. They are related to food, and care practices, and health services (58).
Figure 1
Causes of child malnutrition
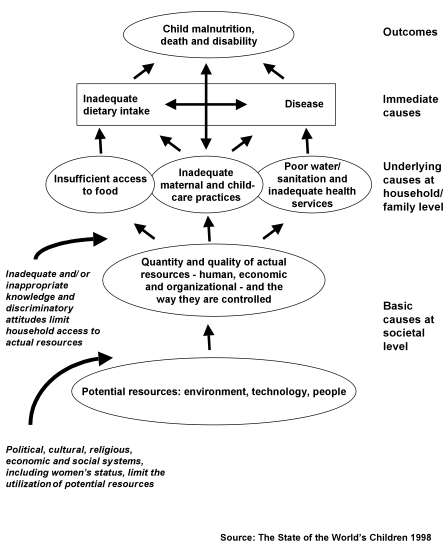
To focus exclusively on food – which is to say, the chemical composition of breastmilk or infant formula – would be to neglect the other two key components, of health and care. For infants and young children, nurturing, the care component, is crucial. It is not just what is fed, but also how, that matters. In infant feeding, close body contact is especially important. While this contact is possible while feeding formula with a bottle, often children are simply given a bottle while the mother attends to other matters, perhaps with the child sucking on a bottle propped up on a pillow. The quality of care is likely to be lower with formula feeding (59).
In some contexts advice is never enough. Rules are needed to protect and facilitate nurturance. The Baby Friendly Hospital Initiative addresses this issue (60-61). Women who have children in prison should be enabled to feed their children and also to nurture them (62). Employed women need to be able to breastfeed at work, and to have their employment paid and protected after up to six months after childbirth. In many cases women's right to be with their infants mist be protected by national or local laws (63).
The complexity of breastmilk
In key respects, formula is grossly inferior to breastmilk. For instance, formula does not include all the immune factors in breastmilk (64). As the March of Dimes points out 'Breast milk includes antibodies and other immune system substances that help protect a baby from illness. It contains growth factors, hormones and other substances that help a baby grow and develop at an appropriate rate. Breast milk also contains fatty acids that appear to promote brain development and, possibly, increase intelligence' (65).
Mechanistic comparisons of ingredients are misleading. These do not convey the complexity of breastmilk (10). Some research suggests benefits from the addition of long-chain polyunsaturated fatty acids to infant formula (66). However, the comparison has been made only between supplemented and unsupplemented formulas. No comparison was made with breastmilk itself. Comparison with breastmilk would be likely to show that both the supplemented and unsupplemented formulas are significantly inferior to breastmilk. Nor has the addition of long-chain polyunsaturated fatty acids to infant formula been shown to produce benefits, either for pre-term infants or full-term infants (67,68). There are good reasons for caution about adding them to the mix.(69-70).
Similar questions arise with other supplements to infant formula, such as prebiotics and probiotics (71). Many are unproven cosmetic changes, apparently designed to exploit parents' willingness to pay higher prices to gain every real or imagined benefit for their children.
Food is more than its chemistry
The UN Codex Alimentarius and the US government judge infant formula to be safe if it includes a basic list of ingredients. This reductionist approach has dangerous consequences. Carlos Monteiro explains: 'Practically all nutritionists now categorise food in terms of its chemical composition, as do most lay writers. This almost universal perception of nutrition is evident in textbooks and scientific journals, and on food labels, journalism, and "diet books". The identification of food with its chemistry is a defining characteristic of modern nutrition science, as invented in the early 19th century' He continues: 'Seeing food in terms of its chemistry has enabled the industrialisation of food systems. In particular, it has made possible the formulation of ultra-processed products from 'refined' or 'purified' chemical constituents of foods – oils, proteins, carbohydrates, and their fractions – together with "micronutrients" – vitamins and minerals'. He summarises: 'Identification of food mainly with its chemical constituents at best has limited value, and in general has provide to be unhelpful, misleading, and harmful to public health' (72).
The regulations for infant formula centre on ensuring that particular components are supplied in specified quantities, an approach that treats formula as a mix of unrelated components. Breastmilk is a complex, changing, living thing, and not simply a collection of inert ingredients. Its complexity is illustrated by the fact that iron in breastmilk is readily available (bioavailable) to the child, but it is not readily available in infant formula. Thus, some manufacturers have included much higher levels of iron in formula than is found in breastmilk. The result can be toxic (73).
There are endless claims to improvements in particular formulas – but little acknowledgment that the previous versions might have been deficient. One study even suggested: 'Addition of human milk proteins to infant formula may be necessary to obtain some of the nutritional and health benefits that breastfed infants enjoy'. It then proposed genetic modification of plants to produce 'recombinant human milk proteins' that can then be added to infant formula (74). The idea that it might be easier, cheaper, and better for infants to be breastfed was not discussed.
There has been no real dispute over the concept that breastfeeding is the gold standard for infant feeding. However, studies frequently make new formulas look good by the simple ruse of comparing new formulas with old formulas, and not comparing either with breastfeeding.
Safety and health are both essential
In the US, the FDA provides regular information on infant formula safety problems such as bacterial contamination with Enterobacter sakazakii (75). These occasional alarms reinforce people's confidence that governments are ensuring the quality of infant formula. However, the FDA offers nothing on long-term failures such as compromised immune systems, or impaired cognitive development, resulting from the use of infant formula. Short-term food safety is not the only thing that matters.
Pharmaceuticals are assessed for both their safety and their effectiveness. Infant formula is not officially a pharmaceutical product, though in many cases the manufacturers are pharmaceutical companies. They do not make explicit claims about effectiveness, but they implicitly claim that infant formula can be regarded as a close approximation to breastmilk in terms of its functionality. Thus, in the US formula is defined as 'a food that purports to be or is represented for special dietary use solely as a food for infants by reason of its simulation of human milk'.
Microbiological safety is not enough
Food standards focus mainly on safety. This leads to narrowing the scope of the concerns considered in setting standards. Thus a study from the US Centers for Disease Control and Prevention claims to be on Food-Related Illness and Death in the United States, but deals only with illness and death associated with specific pathogens in food (76). Focusing on pathogens misses factors like increased risk of colon cancer caused by processed meats, say, or the illnesses and deaths that result from diets high in energy-dense processed products.
Labelling of a food as GRAS ('generally regarded as safe') as the term indicates, depends on whether it is judged to be safe, not on whether it is judged to be nutritionally adequate. GRAS determination does not require any systematic assessment of whether the food is functionally effective. Infant formula may be safe in the sense that it has no E.coli bacteria in it, or that it includes all the ingredients in a prescribed list. But this is far from being able to claim that it has all the elements that contribute to living a full and healthy life. Saying that a food won't make you sick right away is not the same as saying that it meets all your needs.
A whole judgement is needed
Equal attention should be given to nutritional adequacy. What does the product do for the human being who consumes it? The main functionality of infant food and the associated feeding process is to ensure long-term health; not just body-building, which in any case needs to be at a rate to which humans are adapted (77-79), but also protection against infections and allergies, and facilitation of cognitive as well as physical development. Official standards regarding infant formula are attentive to its ingredients, but not to its impact on long-term health and development.
Box 3
What is nutritional adequacy?
The question of the nutritional adequacy of formula has not been properly addressed. Thus one Codex Statement on Infant Formula said: 'Numerous formulae have been produced which offer a nutritionally adequate food for infants'.
But this all depends on what 'nutritionally adequate' means. According to one definition offered by Codex: 'The nutritional adequacy of a product can be defined in terms of protein quality and quantity and content of minerals and vitamins. Such a product should be considered nutritionally equivalent if: (I) its protein quality is not less than that of the original product or is equivalent to that of casein and (ii) it contains the equivalent quantity of protein… and those vitamins and minerals which are present in significant amounts in the original animal products (80).
But it makes no sense to apply this sort of definition to infant formula. A food's nutritional adequacy needs to be assessed in terms of its results, not just its ingredients of macronutrients and micronutrients judged at any time to be most relevant. Infant formula should be viewed as nutritionally adequate only if it is as good for children as breastfeeding. Any other definition shortchanges children.
The evidence is unequivocal. It consistently shows that in any population, formula feeding is consistently worse for children's health than breastfeeding. Judged in this way, there has never been any infant formula that is nutritionally adequate.
The weakness of the standards for infant formula is not surprising. The heavy influence of the transnational and other major infant formula manufacturers in Codex and FDA deliberations is well known (18, 81). The industry successfully presses to keep standards weak. The result is that infants and young children are exposed to unnecessary risks.
Marketing of formula by governments
What's worse, national governments and international agencies distribute formula and thus make it more available and attractive. This is a form of marketing. Some attempts are now made to limit this practice. For example, the idea of sending infant formula into disaster situations has been examined, resulting in stringent limitations on that practice (82,83).
Nevertheless, several national governments persist in distributing infant formula for free, usually to people on low incomes. The UK's Healthy Start programme provides vouchers that can be exchanged for infant formula and other foods. A year's supply of formula is probably worth around £350. For a mother with little available money or support this is a substantial inventive to forget about breastfeeding. When a Member of Parliament asked the relevant government minister why free infant formula is provided, she was told: 'We recommend exclusive breastfeeding for the first six months of life, and the scheme encourages this. However, if mothers choose not to breastfeed, formula is the only safe alternative for children under one year of age, as the use of cow's milk is not recommended' (84). Formula may be safe in a narrow sense, but as argued above, it is not nutritionally adequate. Nor does the mother's preference explain why the government should provide the product at no cost.
In the US the Special Supplemental Nutrition Program for Women, Infants, and Children provides more than half the infant formula used in the country, at no cost to the families. It provides free formula up to the child's first birthday; parents must purchase it after that. As in the UK, this practice serves the interests of the manufacturers by having the government distribute free samples and winning loyalty to their particular brands. It is not in the public interest.
Conclusion
The current system for regulation of infant formula is grossly inadequate. It is based on the assumption that formula is safe and nutritionally adequate. In fact, in settings where water is contaminated it is highly dangerous, and in any setting microbiological contamination is a possibility. Further, formula does not adequately ensure children's health, in the short term and the long term. It lacks many immunoprotective and other substances needed by babies and young children, and is deficient in other ways. Unequivocal evidence showing that formula increases the risk of disease and of death is not taken sufficiently seriously by regulators.
The regulatory system assumes that any formula whose ingredients match up with an established list is safe and nutritionally adequate. The rules are based on the assumption that formula will be used in an optimal way, whereas it often is not. Thus, the issue of water quality is overlooked. Additives are not thoroughly assessed for either their safety or their effectiveness. This approach exposes children to unnecessary risk.
What needs to be done now
The UN Global Strategy for Infant and Young Child Feeding (7) clearly and unequivocally sets out the benefits of extended exclusive breastfeeding until the age of 6 months, followed by complementary breastfeeding until the age of 2 and beyond. It also specifies the benefits of diets for young children based on traditional, local, and culturally appropriate foods. The findings and recommendations of the Strategy have been consistently upheld, most recently at the UN High-Level Meeting on the prevention and control of non-communicable diseases held in New York this September.
The promotion, protection and support of breastfeeding, should be accompanied by public policies that restrict and where necessary prohibit the use of formula. Such measures, introduced incrementally, should make formula less accessible unless when called for by an independent health professional.
National governments should follow the recommendations of the UN High-Level Meeting, and accept their duty to use the law to protect the rights and freedoms of populations and communities including children and their mothers and families. If this is opposed with international regulations being given as a reason, governments should invoke overriding public need as the justification for the use of law to protect their own populations.
In drafting new and stronger laws, regulations and guidelines, governments should work in partnership with health professional and civil society organisations and academicians who have no links with the infant formula and associated industries. The memberships of regulatory bodies need to be reviewed, and all members with links to the formula industry or with any other actual or apparent conflicts of interest should be recused. Once policies and consequent actions are agreed, industry should be invited as partners in their implementation and monitoring.
In such ways public health, public goods, and the public interest, will be well served.
References
- World Cancer Research Fund International (2007). Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington DC: American Institute for Cancer Research, 2007. http://www.dietandcancerreport.org/?p=ER
- US. Department of Health and Human Services. National Women's Health Information Center Breastfeeding—Best for Baby. Best for Mom. Washington DC: DHHS, 2004. http://www.4woman.gov/Breastfeeding/
- American Dietetic Association. Position of the American Dietetic Association: Breaking the barriers to breastfeeding. Journal of the American Dietetic Association 2001. 101(10): 1213-1220.
- McNiel M, Labbok M, Abrahams SW. What are the risks associated with formula feeding? a re-analysis and review. Birth 2010, 37(1): 50-58.
- Wiessinger D. Watch your language! Journal of Human Lactation 1996, 12,(1): 1-4.
- Weimer J.The economic benefits of breastfeeding: a review and analysis. Food and Rural Economics Division, Economic Research Service, US Department of Agriculture Washington DC, 2001.
- World Health Organization/ United Nations Children's Fund. Global Strategy for Infant and Young Child Feeding. WHO, Geneva, 2003.
- Bartick M, Reinhold A. The burden of suboptimal breastfeeding in the United States: a pediatric cost analysis. Pediatrics 2010, 125(5): e1048-56.
- Hale T. Medications and Mother's Milk. 2010: Hale Publishing, Amarillo Texas. Also see http://www.infantrisk.org/
- Lawrence R Breastfeeding: A Guide for the Medical Profession. 7th Edition. 2011: Elsevier Mosby, Maryland Heights, Missouri.
- American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics 2005, 11 (5): 496-506.
- Victora C, Smith P, Vaughan J, Nobre L Lombardi C, Texeira A, Fuchs S, Moreira, L, Gigante L, Barros FC Infant feeding and deaths due to diarrhea: A case control study. American Journal of Epidemiology, 1989, 129: 1032-1034. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2705424&dopt=Abstract
- Chen A, Rogan W. Breastfeeding and the risk of postneonatal death in the United States. Pediatrics, 2004. 113, 5: 435-439.
- Fewtrell M. The long-term benefits of having been breastfed. Current Pediatrics. 2004. 14: 97-103.
- Labbok M, Clark D, Goldman A. Breastfeeding: maintaining an irreplaceable immunological resource, Nature Reviews: Immunology. 2004, 4: 565-572.
- León-Cava N, Lutter C, Ross J, Martin L Quantifying the Benefits of Breastfeeding: A Summary of the Evidence. Washington DC: Pan American Health Organization, 2004. http://www.paho.org/English/AD/FCH/BOB-Main.htm
- Oddy W. The impact of breast milk on infant and child health. Breastfeeding Review 2002, 10,3: 5-18.
- Palmer G. The Politics of Breastfeeding: When Breasts are Bad for Business. Pinter and Martin. Economic Research Report 93, London, United Kingdom, 2009.
- Smith J, Harvey P. Chronic disease and infant nutrition: is it significant to public health? Public Health Nutrition. 2011, 14, 2: 279-289.
- US. Department of Health and Human Services. The Surgeon General's Call to Action to Support Breastfeeding. US Department of Health and Human Services, Office of the Surgeon General, Washington DC, 2011. http://www.surgeongeneral.gov/topics/breastfeeding/index.html
- World Health Organization. Evidence on the Long-Term Effects of Breastfeeding. WHO, Geneva, 2007. http://www.who.int/child_adolescent_health/documents/9241595230/en/index.html
- Wolf J. Low breastfeeding rates and public health in the United States. American Journal of Public Health. 2003, 39, 12: 2000-2010.
- United Nations Children's Fund. Breastfeeding could save 1.3 million infants each year. UNICEF, New York, 2004. http://www.unicef.org/nutrition/index_22657.html
- Richter J. Holding Corporations Accountable: Corporate Conduct, International Codes, and Citizen Action. Zed Books, London, 2001.
- Infant Formula Basics. 2005. Keepkidshealthy.com http://www.keepkidshealthy.com/nutrition/infant_formula_basics.html
- Nommsen-Rivers, Laurie A. Does breastfeeding protect against infant mortality in the United States? Journal of Human Lactation 2004, 20:3, 357-358.
- Codex Alimentarius Commission. Website. http://www.codexalimentarius.net/web/index_en.jsp
- Codex Alimentarius Commission 1976. Statement on Infant Feeding, CAC/MISC-2-1976. http://www.codexalimentarius.net/download/standards/301/CXA_002e.pdf
- Codex Alimentarius Commission 1981. Codex Standard for Infant Formula, Codex Stan 72-1981. http://siweb.dss.go.th/standard/Fulltext/codex/CXS_072E.pdf
- Codex Alimentarius Commission 1985. Code of Ethics for International Trade in Food. CAC/RCP 20-1979 (Rev. 1-1985). http://www.codexalimentarius.net/download/standards/1/CXP_020e.pdf
- World Health Organization. International code of marketing of breast-milk substitutes. 1981: WHO, Geneva. http://www.who.int/nutrition/publications/code_english.pdf Also see subsequent related World Health Assembly Resolutions at http://www.ibfan.org/english/resource/who/fullcode
- Sokol E. The Code Handbook: A Guide to Implementing the International Code of Marketing of Breast-milk Substitutes. Second edition. International Code Documentation Centre, International Baby Food Action Network, Penang, Malaysia, 2005.
- United States Code of Federal Regulations (2004). Title 21, Part 107. Infant formula. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=107
- vLex United States 2011. 21 CFR 107.100 – Nutrient Specifications. http://cfr.vlex.com/vid/107-100-nutrient-specifications-19705849
- Baker R. Commentary: Infant formula safety. Pediatrics 2002, 110:4: 833-835. http://pediatrics.aappublications.org/cgi/content/full/110/4/833
- Statutory Instrument 1997 No. 451: The Infant Formula and Follow-on Formula (Amendment) Regulations. London: HMSO, 1997.
- Statutory Instrument 2003 No. 3208: The Infant Formula and Follow-on Formula (Amendment) (England) Regulations London: HMSO, 2003.
- European Union (1991). Commission Directive 91/321/EEC of 14 May 1991 on infant formulae and follow-on formulae. Official Journal L 175 , 04/07/1991 P. 0035 – 0049.
- US Department of Health and Human Services. Center for Food Safety and Applied Nutrition. US Food and Drug Administration Infant Formula: Overview. USFD, Washington, DC, 2004.. http://www.cfsan.fda.gov/~dms/inf-toc.html
- American Academy of Pediatrics. Soy protein-based formulas: recommendations for use in infant feeding. Pediatrics 1998, 101,1: 148-153. http://aappolicy.aappublications.org/cgi/reprint/pediatrics;101/1/148.pdf
- Merritt R, Jenks B. Safety of soy-based infant formulas containing isoflavones: The clinical evidence. Journal of Nutrition.2004, 134: 1220S-1224S. http://jn.nutrition.org/cgi/content/abstract/134/5/1220S
- Agricultural Research Service. Study examines long-term health effects of soy infant formula. Agricultural Research 2004, 52,2: 8-10. http://www.ars.usda.gov/is/AR/archive/jan04/soy0104.pdf
- Badger T, Gilchrist J, Pivik R, Andres A, Shankar K, Chen; J, Ronis M. The health implications of soy infant formula. American Journal of Clinical Nutrition 2009, 89,5: (suppl)1668S-1672S. http://www.ajcn.org/content/89/5/1668S.full
- British Dietetic Association.. Paediatric group position statement on the use of soya protein for infants. Journal of Family Health Care 2003,,4: 93. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14528647&dopt=Abstract
- Soy On Line Service website. http://www.soyonlineservice.co.nz/
- American Academy of Pediatrics). Use of soy protein-based formulas in infant feeding. Pediatrics 2008, 121: 1062-1068. http://pediatrics.aappublications.org/cgi/content/abstract/121/5/1062
- FDA scientists questions soy safety - but where is GM testing? (2004). http://www.netlink.de/gen/Zeitung/2000/000609.html
- Barnett A. They hailed it as a wonderfood: soya not only destroys forests and small farmers—it can also be bad for your health. The Observer 7 November 2004.. http://observer.guardian.co.uk/foodmonthly/story/0,9950,1342291,00.html
- Heinz Goes Green, Guarantees Baby Food GMO-free (2003). CTV.ca Jan 31. http://www.ctv.ca/servlet/ArticleNews/story/CTVNews/1043968381082_39377581/
- Sarmiento R. Wyeth products now free of GMO, says Greenpeace. Philippines Today, 8 August 2003. http://www.bic.searca.org/news/2003/aug/phi/08.html
- Lane Y (2010). GM protestors want baby formula out. International Business Times. 28 September 2010. http://au.ibtimes.com/articles/65765/20100927/gm-protesters-want-baby-formula-out.htm
- Greenpeace and INFACT Canada.Survey Results of Genetically Engineered (GE) Infant Formula and Baby Foods, 29 Ocrober 2002. http://www.healthcoalition.ca/survey-babies.pdf
- These statements are no longer on IDFA's website, but there are copies at http://www.babymilk.com/children/index.htm and several other websites.
- US Department of Health and Human Services. Center for Food Safety and Applied Nutrition. U.S. Food and Drug Administration. Guidelines concerning notification and testing of infant formulas. FDA, Washington DC, 1985. http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/InfantFormula/ucm169730.htm
- Institute of Medicine. Committee on the Evaluation of the Addition of Ingredients New to Infant Formula. Infant formula: Evaluating the safety of new ingredients. Washington, D.C.: National Academies Press, 2004. http://www.iom.edu/report.asp?id=19034
- Stehlin I. Infant formula: second best but good enough. FDA Consumer Magazine. June, FDA, Washington, D.C, 1996. http://www.fda.gov/fdac/features/596_baby.html
- Martin A. Consumer agency tightens scrutiny of baby sleep products. New York Times. 31 January 2011. http://www.nytimes.com/2011/02/01/business/01safety.html?ref=andrewmartin
- Pelletier D. Toward a Common Understanding of Malnutrition: Assessing the Contributions of the UNICEF Framework. UNICEF/World Bank, New York/Washington DC, 2002.
http://www.tulane.edu/~internut/publications/WB_Bckgrd_Pprs/Narrative/NarrativeonePelletierfinal.doc - Smith J, Ellwood M Feeding Patterns and Emotional Care in Breastfed Infants. Social Indicators Research., 2009. http://www.springerlink.com/index/V6V8227758T65R30.pdf
- Baby-Friendly USA, Inc. Website. http://www.babyfriendlyusa.org/
- United Nations Children's Fund. The Baby-Friendly Hospital Initiative. Website. UNICEF, New York. http://www.unicef.org/programme/breastfeeding/baby.htm
- Women's Prison Association (2009). Mothers, Infants and Imprisonment: A National Look at Prison Nurseries and Community-Based Alternatives. WPA, New York. http://www.wpaonline.org/pdf/Mothers%20Infants%20and%20Imprisonment%202009.pdf
- Dugger C. Where a cuddle with your baby requires a bribe. New York Times, 30 August 2005. http://www.nytimes.com/2005/08/30/international/asia/30bangalore.html
- Hanson L Immunobiology of Human Milk: How Breastfeeding Protects Infants. Pharmasoft Publishing, Amarillo, Texas, 2004.
- March of Dimes (2004). Breastfeeding. http://www.marchofdimes.com/pnhec/188_1061.asp
- Willatts P, Forsyth J, Di Modugno MK, Varma S, Colvin M. Effects of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet 1998, 352, 9129: 688-692.
- Simmer K, Schulzke S, Patole S. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database of Systematic Reviews, 2008. http://www2.cochrane.org/reviews/en/ab000375.html
- Simmer K, Patole S, Rao S. Longchain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database of Systematic Reviews, 2008. http://www2.cochrane.org/reviews/en/ab000376.html
- Cornucopia Institute. Replacing Mother – Imitating Human Breast Milk in the Laboratory. CI, Cornucopia, Wisconsin, 2008. http://www.cornucopia.org/2008/01/replacing-mother-infant-formula-report/
- Cornucopia Institute. DHA and ARA in Infant Formula: Dangerous and Unnecessary—Synthetic Additives Have No Place in Infant Foods. CI, Cornucopia, Wisconsin, 2010.. http://cornucopia.org/DHA/DHA-Update-2010.pdf
- Shafai T. Routine supplement of prebiotics and probiotics to newborn infants are not recommended [Letter]. Pediatrics 2009. 123; e543-e544. http://pediatrics.aappublications.org/cgi/content/full/123/3/e543
- Monteiro C. The big issue is ultra-processing. 'Carbs': The answer. World Nutrition 2011. 2, 2: 86-97. http://wphna.org/2011_feb_wn4_cam5.htm
- Lönnerdal B, Kelleher S Iron metabolism in infants and children. Food and Nutrition Bulletin. 2007,3 Suppl: S491-499. http://foodandnutritionbulletin.org/FNB/index.php/FNB/article/view/1903/1916
- Lönnerdal B. Expression of human milk proteins in plants. Journal of the American College of Nutrition 2002, 21, 3: 218S-221S. http://www.jacn.org/cgi/reprint/21/suppl_3/218S
- US. Food and Drug Administration. Food Safety. Product Specific Information. Website. FDA, Washington, DC. http://www.fda.gov/Food/ FoodSafety/Product-SpecificInformation/default.htm
- Mead P, Slutsker L, Dietz V, McCaig L, Bresee, J, Shapiro C, Griffin P, Tauxe R. Food-related illness and death in the United States. Emerging Infectious Diseases. 1999, 5, 5: 607-625. http://www.cdc.gov/ncidod/eid/vol5no5/mead.htm
- Garza C, de Onis M. A new international growth reference for young children. American Journal of Clinical Nutrition. 1999, 70, (1): 169S-172S. http://www.ajcn.org/cgi/content/full/70/1/169S?maxtoshow=&HITS=10&hits=10&RESULTFORMA
- De Onis M, Garza C, Victora C, Onyango A, Frongillo E, Martines J. The WHO Multicentre Growth Reference Study (MGRS): planning, study design, and methodology. Food and Nutrition Bulletin 2004, (1 Suppl) S15-26. http://www.ncbi.nlm.nih.gov/pubmed/15069916
- Fomon, Samuel J. Assessment of growth of formula-fed infants: evolutionary considerations. Pediatrics. 2004, 113, (2): 389-393.
- Codex Alimentarius Commission.Codex General Guidelines for the Utilization of Vegetable Protein Products (VPP) in Foods. CAC/GL 4-1989.
- Koletzko B, Shamir R. Editorial: Standards for infant formula milk: commercial interests may be the strongest driver of what goes into formula milk. British Medical Journal. 2006, 16, 332: 621. http://www.bmj.com/content/332/7542/621.extract
- Wellstart International . Infant and Young Child Feeding in Emergency Situations. WI, San Diego, California, 2005. http://www.wellstart.org/Infant_feeding_emergency.pdf
- World Health Organization. Infant and Young Child Feeding in Emergencies: Operational Guidance for Emergency Relief Staff and Programme Managers. Version 2.1 WHO, Geneva, 2007.
http://www.who.int/entity/nutrition/publications/emergencies/operational_guidance/en/index.html - NCT Watch. Minister questioned about the inclusion of infant formula in Healthy Start. National Childbirth Trust, London, 2009. http://nctwatch.wordpress.com/2009/03/27/minister-questioned-about-the-inclusion-of-infant-formula-in-healthy-start/
Acknowledgement and request
Readers are invited please to respond. Please use the response facility below. Readers may make use of the material in this editorial if acknowledgement is given to the Association, and WN is cited
Please cite as: Kent G. Breastfeeding. The need for strong regulation to protect the health of babies. [Commentary] World Nutrition, September 2011, 2, 9 465-490. Obtainable at www.wphna.org
The opinions expressed in all contributions to the website of the World Public Health Nutrition Association (the Association) including its journal World Nutrition, are those of their authors. They should not be taken to be the view or policy of the Association, or of any of its affiliated or associated bodies, unless this is explicitly stated.
WN commentaries are subject to internal review by members of the editorial team.
This commentary is adapted from my book on Regulating Infant Formula, to be published by Hale Publishing of Amarillo, Texas. Many thanks to James Akré, Carol Bartle, Stephen Buescher, Arly Helm, Pamela Morrison, Touraj Shafai, Julie Smith, Virginia Thorley, Marian Tompson, and Margret Vidar.